Mol Calculator User Manual
V2.08
Appendix 1. Classification of data
Revision history
|
V1.00 |
Initial release. |
|
V1.01 |
Corrected the text error of formula. Coef(1) x n(1) = Coef(2) x n(2) = Coef(3) x n(3) = Coef(4) x n(4) = Coef(5) x n(5) -> n(1)/Coef(1) = n(2)/Coef(2) = n(3)/Coef(3) = n(4)/Coef(4) = n(5)/Coef(5) Executable code is the same as V1.00. |
|
V2.00 |
Added the following
buttons: P_Pres button, G_Vol button,
and PresVol selector button. Extended so that
either pressure data or volume data belongs to P button data group. |
|
V2.01 |
Modified the volume of
gas at the standard temperature and pressure (0 ℃, 1 atm) to 22.414
L from 22.4 L. |
|
V2.02 |
Added description
about the integrity of displayed data. |
|
V2.03 |
Corrected the
character string in help to "22.414" from "22.4". |
|
V2.04 |
Corrected pressure
output data at [kPa] and [mmHg] unit for the pressure mode P button calculation. |
|
V2.05 |
Updated for iOS7
and the new SDK of Xcode5. The status bar was
hidden. |
|
V2.06 |
Updated for iOS8
and the new SDK of Xcode6. |
|
V2.07 |
Updated for iOS9 and
the new SDK of Xcode7. |
|
V2.08 |
Updated for iOS10 and
the new SDK of Xcode8. Added explanation to
the PresVol mode and the P button data group |
1. Overview
Mol calculator is a calculation sheet that solves chemical mol calculation problems.
A typical problem is as follows.
(Example)
The hydrogen gas used for a fuel cell is
generated from aluminum powder particles and water. (1) When the temperature is
20 ℃ and the pressure is 1
atmosphere, how much liters of hydrogen gas can be generated from 1 g of
aluminum?
(2) When the gas is generated in a closed container of 0.1 liter at the
temperature of 20 ℃, how much is the pressure of hydrogen gas?
(1) Volume calculation
Mol calculator solves the problem in the following three steps.
<Step 0: Preparation>
Prepare chemical equations and atomic (molecular) weights. They are basics for the calculation.
They are as follows.
2Al + 6H2O -> 2Al(OH)3 + 3H2
Atomic weight of Al = 27 [g/mol]
Molecular weight of H2 = 2 [g/mol]
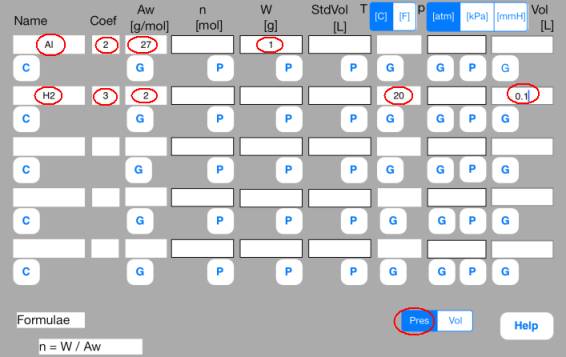
<Step 1: Definition>
Enter known data into the Mol sheet.
Rows correspond to substances. Columns correspond to the attributes of substance.
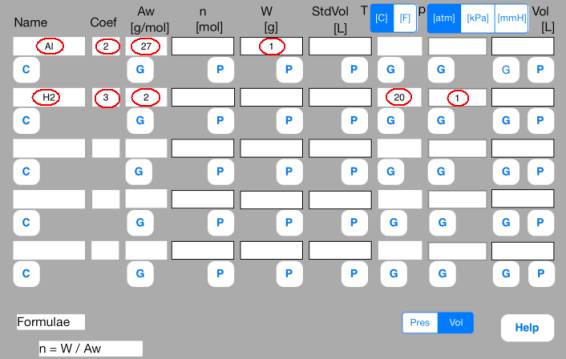
First row
Name = Al, Coef = 2, Aw = 27, W = 1
Second row
Name = H2, Coef = 3, Aw = 2, T = 20[C], p = 1[atm]
<Step 2: Calculation>
Attribute data are not independent but mutually related by formulae.
Calculation is a conversion from unknown data to known data using the formulae.
The formulae are embedded in P and G buttons.
Touching out those buttons executes the formulae and changes unknown data to known data.
Touch out buttons as follows.
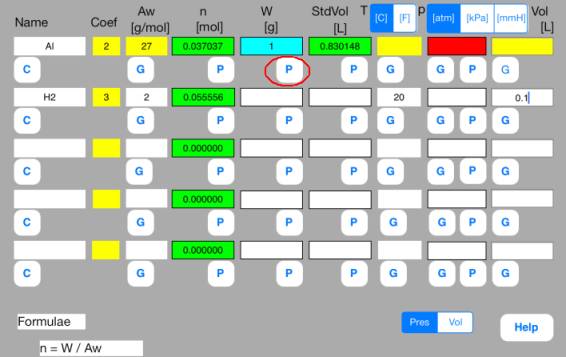
Touch out P_W button of the first row. Mol data are changed to known data.
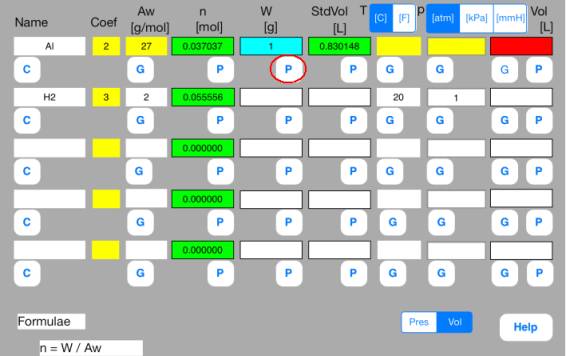
Touch out P_Mol button of the second row. Vol data are changed to known data.
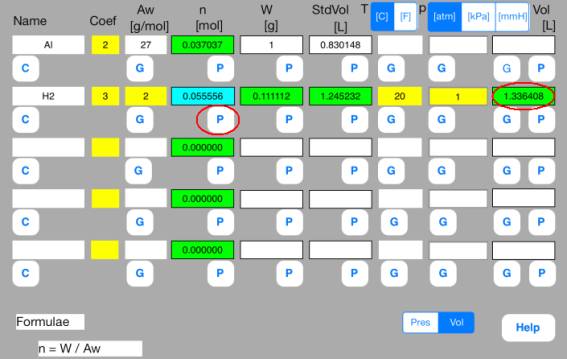
It turns out that the volume of hydrogen gas is 1.3 L.
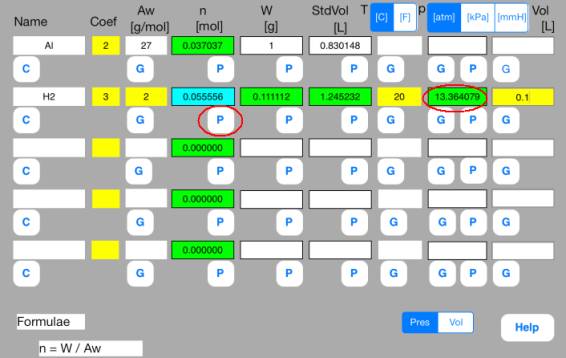
(2) Pressure calculation
<Solution by
V1.00>
Use the above results.
Enter the following data
in the second row.
Vol
= 0.1[L]
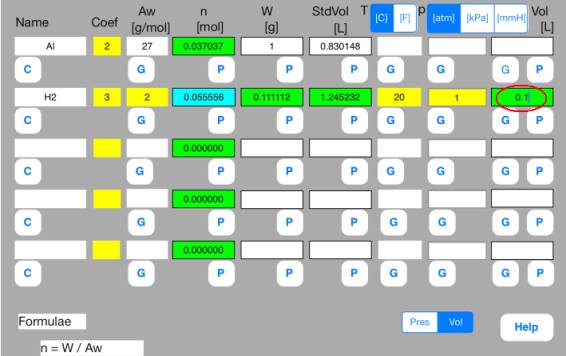
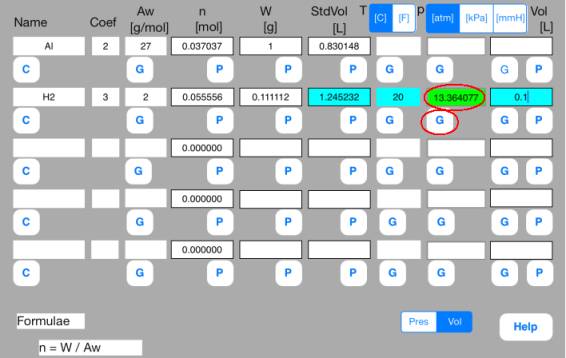
Touch out G_Pres button
of the second row. Pressure data are changed to known data.
<Step 1:
Definition>
Enter the following
data.
First row
Name
= Al, Coef = 2, Aw = 27, W = 1
Second row
Name
= H2, Coef = 3, Aw = 2, T = 20[C], Vol = 0.1[L]
2. Data label
Name: Substance name (option).
Coef: Substance coefficient of chemical equation.
This defines quantity ratio among substances.
When a chemical equation is aX + bY -> cZ, then a, b, and c are coefficients.
When the Coef text of the data field is not numeric, it is interpreted as zero value.
Aw [g/mol]: Atomic or molecular weight of substance
n [mol]: Mol quantity of substance.
W [g]: Weight of substance.
StdVol [L]: Gas volume of substance at 0 deg C and 1 atm.
T [C][F]: Temperature.
p [atm][kPa][mmHg]: Gas pressure.
Vol [L]: Gas volume of substance at the temperature T and at the pressure p.
3. Buttons and data
<P button and P button data group>
P (put) button inputs data to which the P button is assigned.
P button data group is a group of data to which the P buttons are assigned. The P button data group has a special character. When one P data is input, all other data of the P button data group are updated by formulae. When the P button is touched out, Mol data of other rows are also updated by use of Coef data.
When the data field is blank, it is interpreted as illegal data.
When the data field is illegal character, it is interpreted as zero value.
Number of input data: number of output data = 1: N, where N > 1.
G (get) button gets data from other data using formulae.
When the data field is blank, it is interpreted as illegal data.
When the data field is illegal character, it is interpreted as zero value.
Number of input data: number of output data = N: 1, where N > 1.
The manual modification
of data may break the integrity of displayed data. However, G_button and
P_button executions performed succeeding later recover the integrity of
displayed data.
<C button>
C (Clear) button clears all row data.
<PresVol button>
Either pressure data or
volume data belongs to P button data group.
See Appendix 1.
The PresVol button
specifies pressure mode or volume mode.
In pressure mode,
pressure data belongs to P button data group. And, the P_Pres button is
displayed.
In volume mode, volume
data belongs to P button data group. And, the P_Vol button is displayed.
The default mode is
volume mode.
<PresVol mode and P
button data group>
P button data represents
substance quantity.
PresVol mode determines
the P button data group.
In the Pres mode,
elements of the P button data group are n[mol], W[g], StdVol[L], and
p[atm][kPa][mmHg].
In the Vol mode,
elements of the P button data group are n[mol], W[g], StdVol[L], and Vol[L].
The difference is below.
In the Pres mode,
p[atm][kPa][mmHg] is an element of the P button data group ..
In the Vol mode, Vol[L]
is an element of the P button data group .
4. Color change
Touching out a button executes calculation and generates output data from the input and the referenced data. Unknown data are changed to known data.
Color change shows what data are input, output, or referenced data in this button execution.
Input data: Blue color
Referenced data: Yellow color
Output data: Green color
Uncalculated data: Red color
Referenced data is an input data that is referenced at the P button execution.
Uncalculated data is an output data that is not normally calculated. This uncalculated data is not output. The former data is remains unchanged in this data field.
5. Formulae
Embedded formulae are as follows.
n = W / Aw
StdVol = 22.414 x n
StdVol /273.15 = p x Vol / (T + 273.15)
n(1)/Coef(1) =
n(2)/Coef(2) = n(3)/Coef(3) = n(4)/Coef(4) = n(5)/Coef(5)
6. Features
This software solves the problem not as a black box but as a white box. You need to think the process of solving a problem. This makes your knowledge refresh. The black box solution is convenient; however it has a risk of losing your knowledge. This software has few such risks.
7. Examples
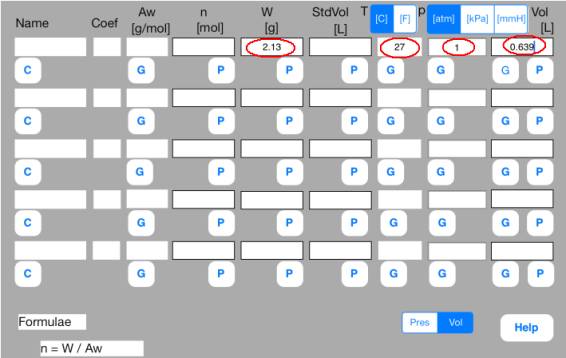
(Example-1) Calculation of molecular weight
There is 639 mL of gas at the temperature 27 degC and the pressure 1 atom.
The weight of the gas is 2.13 g. How much is the molecular weight of the gas?
<Step 0: Preparation>
Gather known data.
<Step 1: Definition>
Enter known data into the Mol sheet.
First row
W = 2.13, T = 27[C], p = 1[atm], Vol = 0.639[[L]
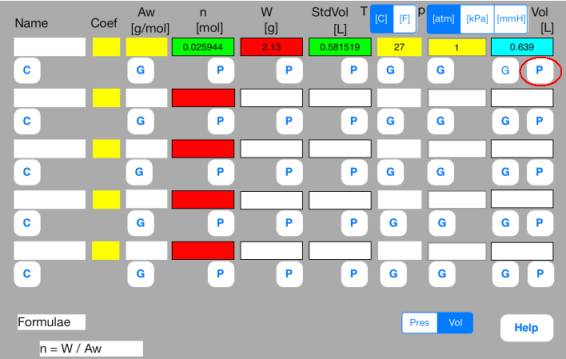
<Step 2: Calculation>
Touch out P_Vol button of the first row. Mol data are changed to known data.
Touch out G_Aw button of the first row. Atomic weight (molecular weight) data are changed to known data.
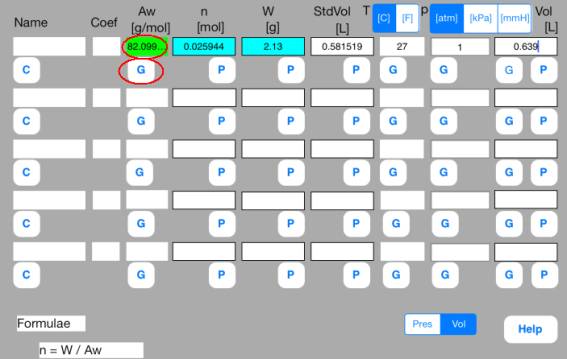
It turns out that the molecular weight of the gas is 82 g/mol.
Appendix
1. Classification of data
Physical and chemical
data are classified into non-substance data and substance data.
The non-substance data
is a data that does not depend on substance.
(Example) Time, voltage
The substance data is a data
that depends on substance, i.e., that is property data of substance.
Moreover the substance
data is classified into intensive data and extensive data.
The intensive data is a data
that does not depend on substance quantity.
(Example) Atomic weight, density, concentration
The extensive data is a data
that depends on substance quantity.
(Example) Mol, weight,
volume, charge
The P button data group of
this software corresponds to the extensive data group.
This classification varies
with application.
For the gas within a
balloon container, the gas pressure is considered to be the intensive data and
the volume is considered to be the extensive data.
For the gas within an
airtight container, the gas pressure is considered to be the extensive data and
the volume is considered to be the intensive data.